The aim of the EuroSpA Research Collaboration Network
The aim of the EuroSpA Research Collaboration Network (RCN) is to jointly address research questions in SpA including, but not limited to, real world effectiveness and safety profiles of biologic therapies. This is done by retrospectively and prospectively collecting real life patient data from European registers.
The collaboration promotes opportunities to identify unmet needs in the treatment of SpA patients by utilizing high-quality data from existing patient registries.
Our aims are in line with the EMA initiative for patient registries and are consistent with discussions at the EMA workshop “How to make better use of patient registries to collect high-quality data on medicines” held in London on October 28th, 2016 in London.
EuroSpA RCN Scope
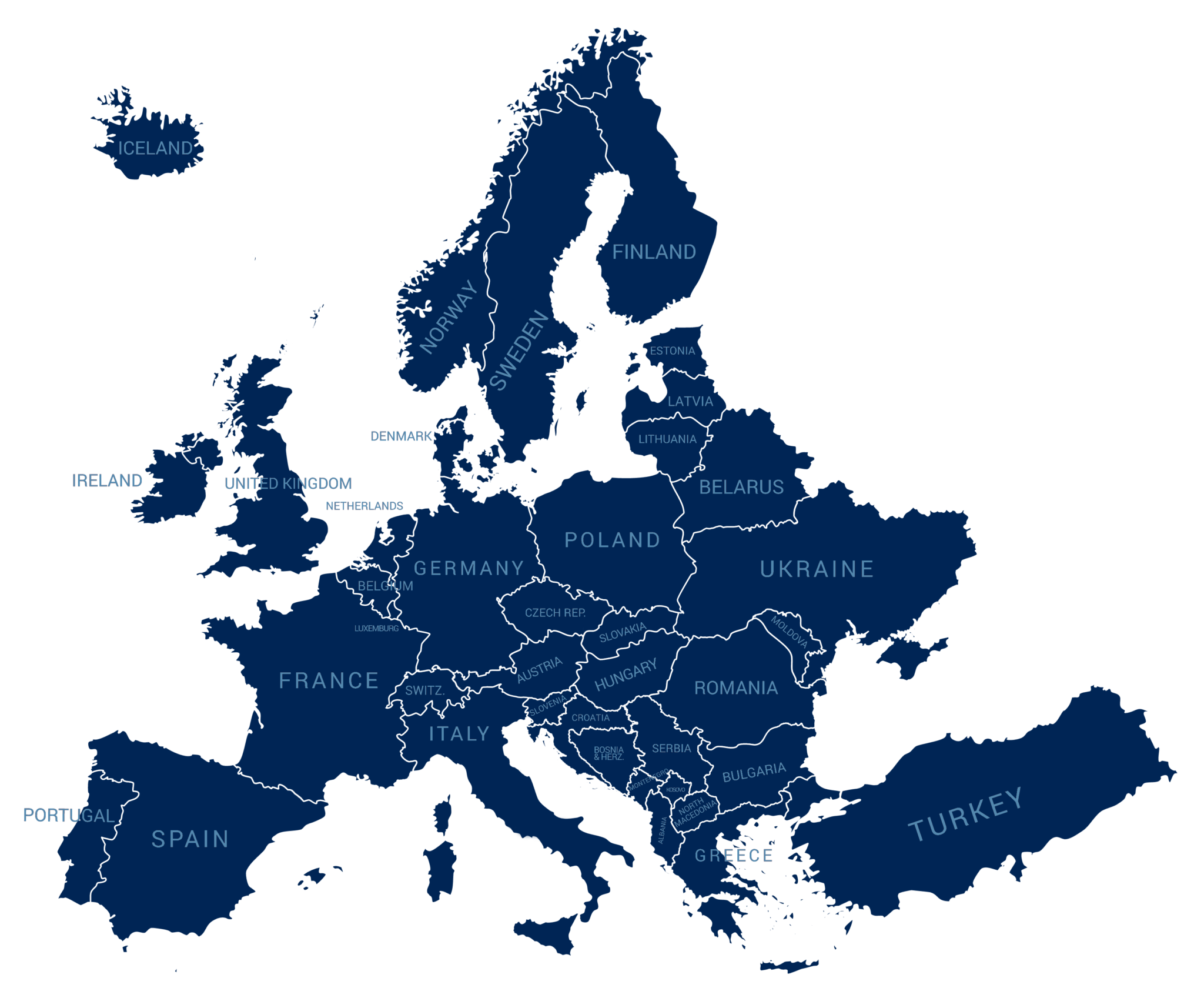
Currently the collaboration consists of 17 registries (see map).
There is a recognized openness to include other interested registries and to other parties such as health authorities (EMA and local regulatory bodies) and patient organizations, although the extent of their involvement will need to be further defined.
Acknowledgements
Novartis Pharma AG for supporting the EuroSpA collaboration.
UCB Biopharma SRL for supporting the EuroSpA collaboration.
Registries that contribute data to the collaboration
ARC (Netherlands)
ATTRA (Czech Republic)
BIOBADASER (Spain)
Biorx.si (Slovenia)
BSRBR-AS (United Kingdom)
DANBIO (Denmark)
ERSBTR (Estonia)
GISEA (Italy)
ICEBIO (Iceland)
NOR-DMARD (Norway)
RABBIT-SpA (Germany)
Reuma.pt (Portugal)
ROBFIN (Finland)
RRBR (Romania)
SCQM (Switzerland)
SRQ (Sweden)
TURKBIO (Turkey)


































The most recent EuroSpA publications
EuroSpA publication D3.1
Enthesitis in a European registry-based cohort of patients with psoriatic arthritis treated with tumour necrosis factor inhibitors: clinical burden, patient-reported outcomes, and treatment response [...]
EuroSpA publication 1.3
Drug effectiveness of 2nd and 3rd TNF inhibitors in psoriatic arthritis – relationship with the reason for withdrawal from the previous treatment [...]
EuroSpA publication 2.1
Patient-reported outcomes and PRO remission rates in 12,262 biologic-naïve patients with psoriatic arthritis Click here to access full article [...]
EuroSpA publication D1.1
Sex Differences in the Effectiveness of First-Line Tumor Necrosis Factor Inhibitors in Psoriatic Arthritis: Results From the European Spondyloarthritis Research Collaboration Network [...]
EuroSpA publication A1.1
Patient-reported outcomes in axial spondyloarthritis and psoriatic arthritis patients treated with secukinumab for 24 months in daily clinical practice Click here to [...]
To see full list, please click Publications in top menu
EuroSpA Research Collaboration Network explained by chairpersons Professor Merete Lund Hetland and Professor Mikkel Østergaard
Why is it meaningful to be a part of EuroSpA? – an interview with 8 members of the Scientific Committee
EuroSpA meetings
EuroSpA Scientific Committee consists of representatives from the 17 participating registries, who meets for hybrid meetings twice yearly in Copenhagen and online.
